Bioprocess Development
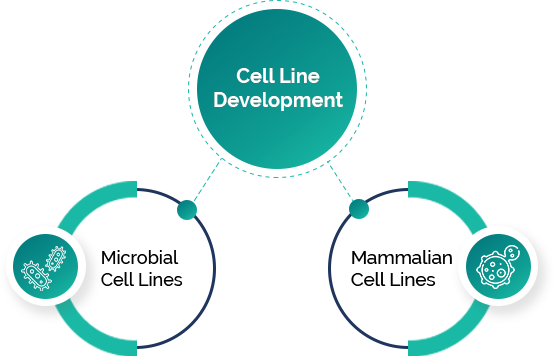
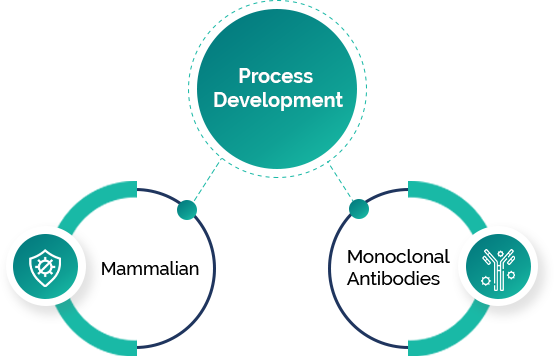
Veeda Biopharma’s Bioprocess Development team specializes in the development of therapeutic and non-therapeutic proteins using microbial (E. coli) and mammalian (CHO; HEK) expression systems. We focus on creating high-yield, stable clones with comprehensive traceability throughout the process. Starting at the microbioreactor level, we scale up production in 5 L bioreactors with the capability to expand to a 50 L single-use bioreactor, strictly adhering to GMP and GLP standards.