We conduct Phase I clinical trials to assess the safety and tolerability of new treatments in participants, ensuring a solid foundation for further testing.
In Phase II and III clinical trials, we evaluate the efficacy and optimal dosing of treatments in larger patient groups, gathering crucial data for regulatory approval.
After approval, our Phase IV clinical trials monitor the long-term safety and effectiveness of treatments in real-world settings, providing valuable insights for ongoing patient care.

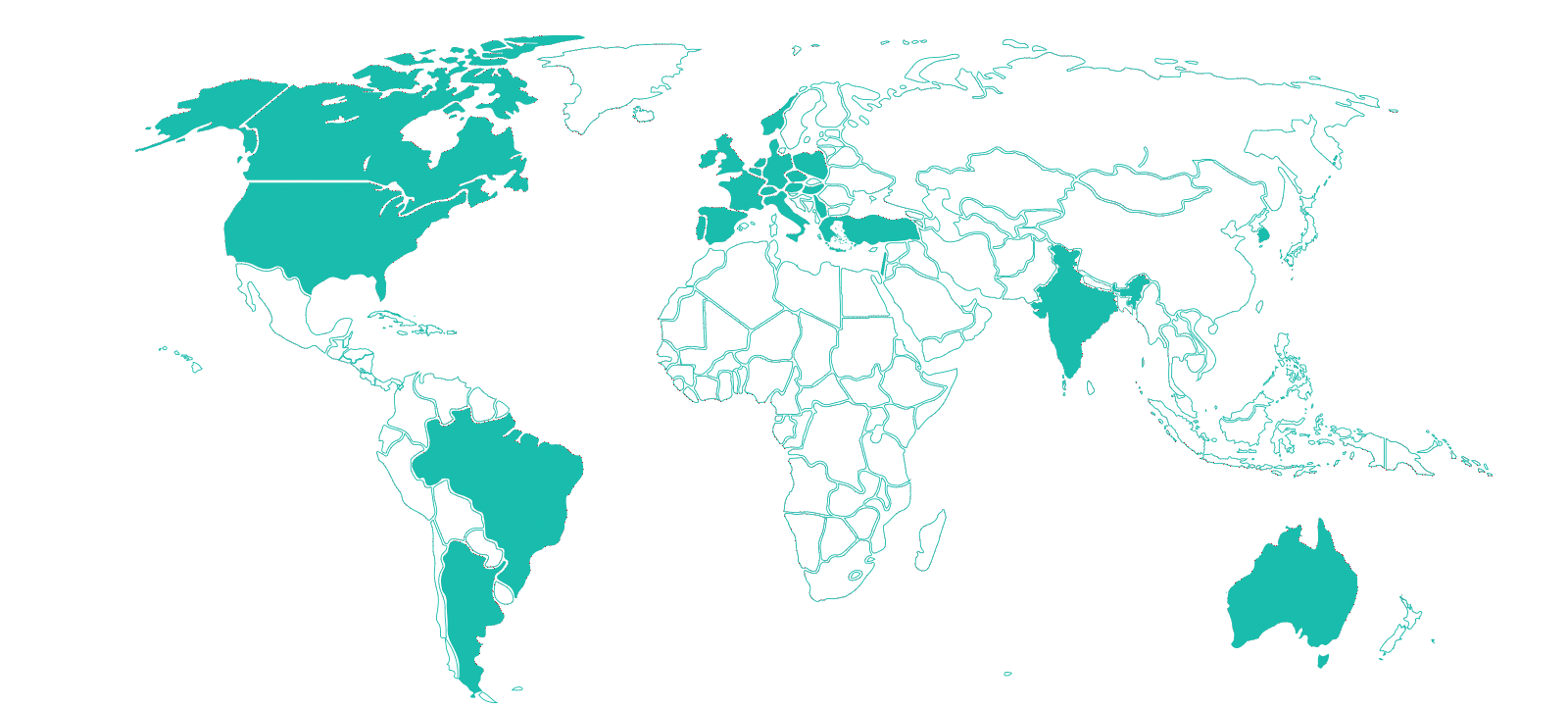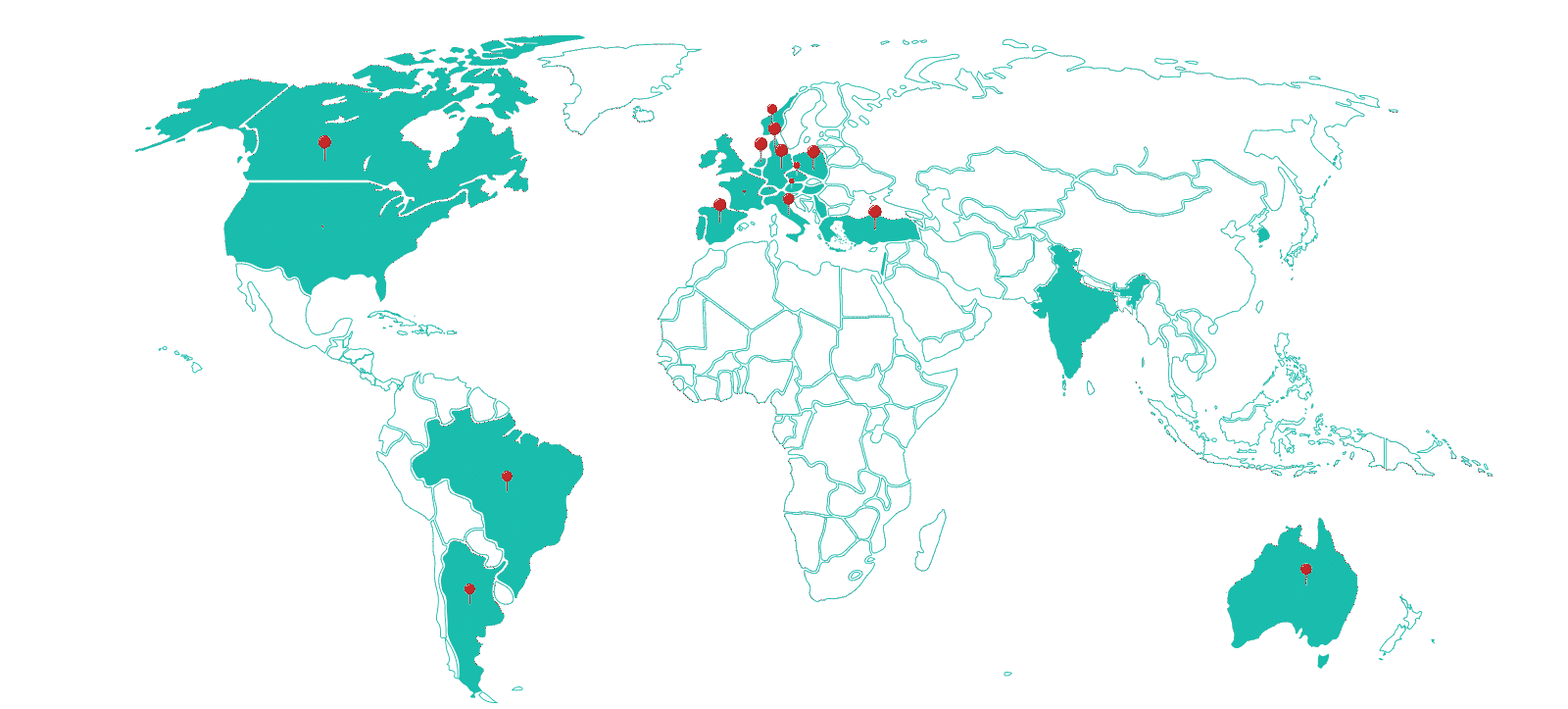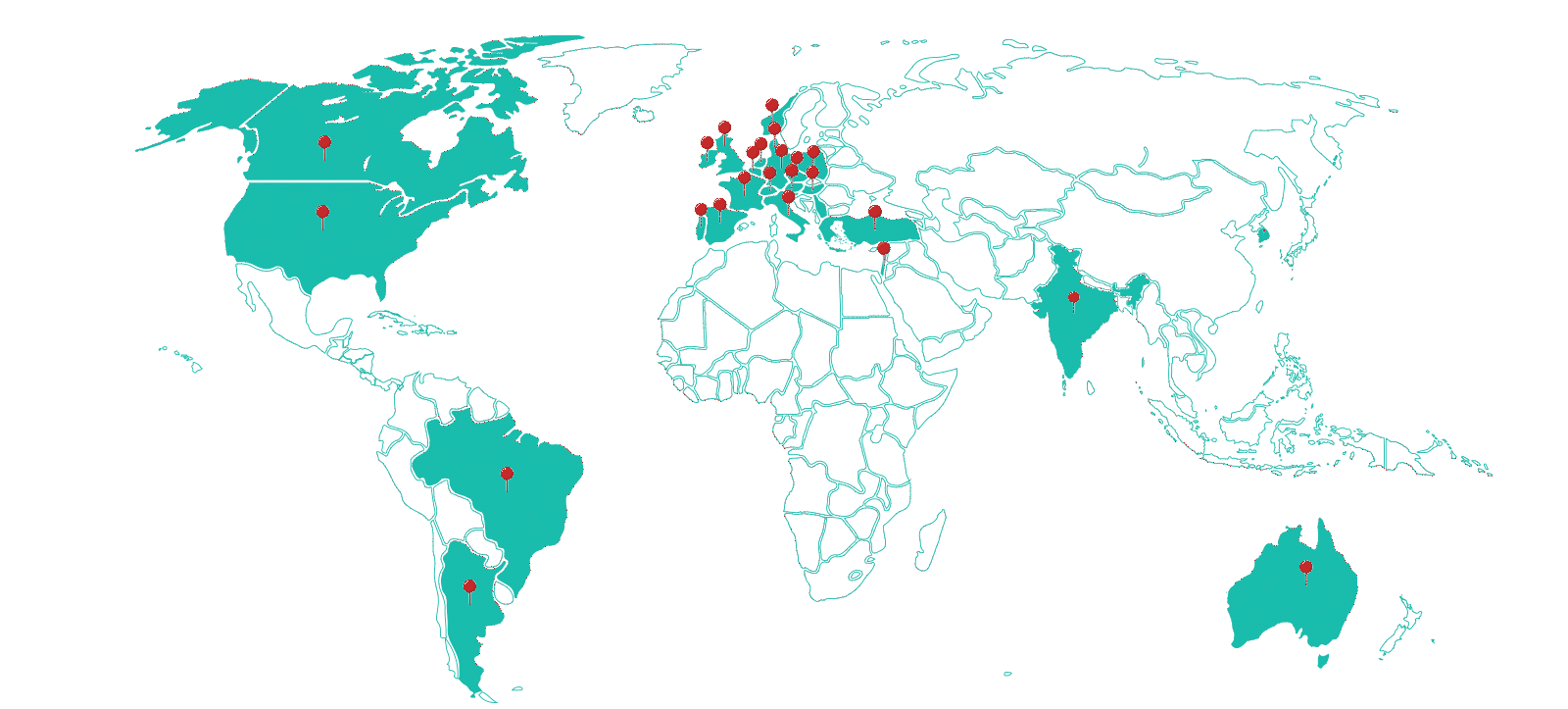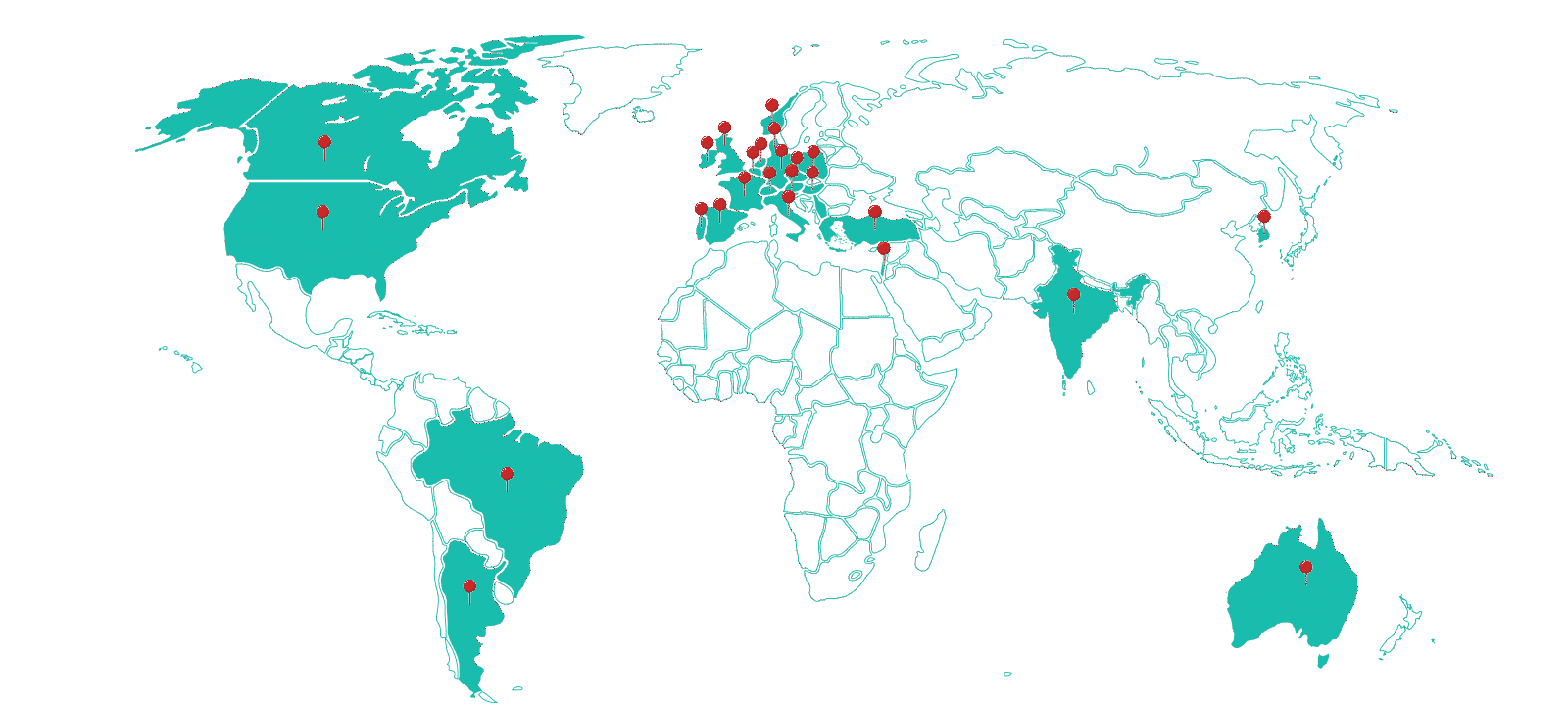
Therapeutic Areas
Sites
Investigators Database
Clinical Trials Supported
Patients Enrolled
Our Clinical Trial Management System (CTMS) gives you a centralized and up-to-date database for your studies, providing real-time visibility and control. You can easily map out the entire clinical trial lifecycle—from recruitment to reporting—helping your research team stay on top of all necessary activities.
A digital platform strategy is essential for effectively capturing, organizing, sharing, and storing the vital documents, images, and artifacts that emerge during the lifecycle of a regulated clinical trial. These platforms streamline workflows and enhance collaboration among stakeholders, ensuring that all critical information is easily accessible and up-to-date.

Chief Operating Officer, Global Clinical Trials
“Every patient’s journey is unique, and so is our approach to clinical trials. We reduce the complexity of research by designing solutions that prioritize patients—optimizing site selection, accelerating recruitment, and tailoring engagement strategies to ensure a meaningful and seamless trial experience. Together, we can create trials that not only advance science but also truly serve the people at the center of it.”

Our skilled clinical research associates (CRAs) ensure smooth site interactions and conduct thorough data reviews. They provide essential support in site selection and monitoring, making sure that your clinical trials run smoothly and follow the protocol.
While data collection forms the foundation of the study, we also place the patient at the center of the trial process. Our patient recruitment strategy for clinical studies is focused on identifying the right participants, ensuring their willingness to take part, and maintaining a strong pool of committed individuals. Our commitment to participant retention is key to helping you conduct successful studies.
While data collection forms the foundation of the study, we also place the patient at the center of the trial process. Our patient recruitment strategy is focused on identifying the right participants, ensuring their willingness to take part, and maintaining a strong pool of committed individuals. Our commitment to participant retention is key to helping you conduct successful studies.

Expert insights into the latest advancements, evolving treatment strategies, and the future of care for patients.

From preclinical to late phase trials, Veeda Group combines advanced technology, global expertise, and agility to streamline every stage of drug development.
A European research-centered pharmaceutical company was seeking marketing approval for its Leuprolide acetate 30 mg drug candidate.