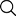Articles


Global Asthma Crisis: Innovation in Drug Development
Asthma has emerged as a formidable global health challenge, evolving from a manageable chronic condition to the second leading cause of death among chronic respiratory diseases.
The alarming trajectory shows asthma cases surging from 287 million in 1990 to 339 million in 2023, with projections indicating 520 cases per 100,000 people by 2050 (Figure 1).
This exponential growth, driven by urbanization, air pollution, climate change, and demographic shifts, has created an urgent need for innovative therapeutic solutions and robust clinical research capabilities.
Comprehensive analysis of the global asthma market showing disease burden trends, regional market growth projections, and therapeutic class valuations across key timeframes.
The pharmaceutical response to this crisis reflects both opportunity and necessity. The global asthma drugs market, valued at USD 27.42 billion by 2025, is projected to reach USD 36.49 billion by 2030.
However, this market expansion coincides with increasingly complex regulatory landscapes in key markets like the United States and Europe, where stringent standards demand sophisticated clinical research partnerships.
GLOBAL ASTHMA MARKET ANALYSIS
Figure: 1
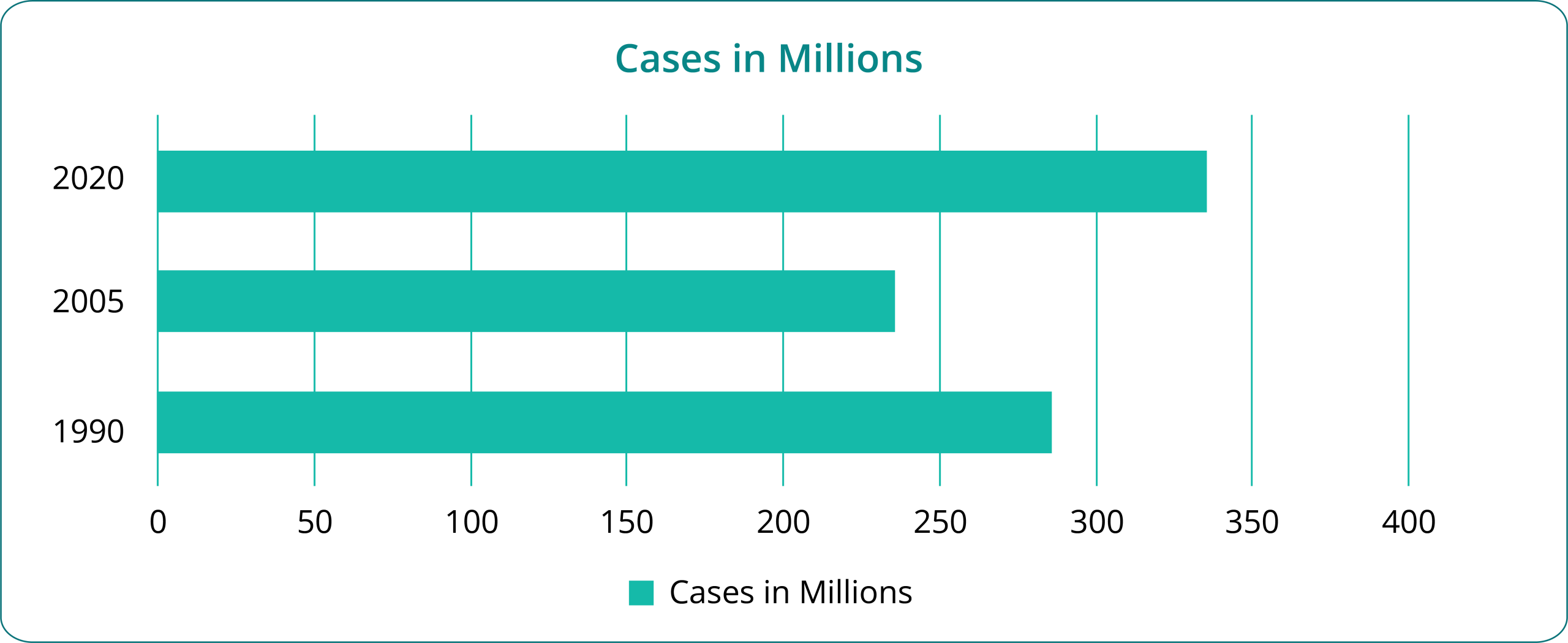
Figure 2: Growth of Different Drug Classes Supporting Asthma ($b)
Inhaled Corticosteroids (ICS); Long-Acting Beta Agonists (LABA); Leukotriene Receptor Antagonists (LTRAs); Short-Acting Beta Agonists (SABAs); Monoclonal Antibodies (mAbs).
Veeda Lifesciences: A Proven Partner in Respiratory Drug Development
Comprehensive Inhalation Expertise and Regulatory Excellence
Veeda Lifesciences has established itself as a leading independent full-service Contract Research Organization (CRO) with specialized expertise critical for asthma drug development.
The company’s capabilities span from preclinical research through Phase IV clinical trials, positioning it as an ideal partner for pharmaceutical companies navigating the complex landscape of respiratory therapeutics.
The organization’s inhalation clinical trials expertise represents a particular strength in asthma drug development.
Veeda has demonstrated proficiency in addressing the unique challenges of inhalation studies, including device compatibility, patient training, and the complex pharmacokinetic considerations essential for respiratory medications.
With over 5000 BA/BE studies, including 50 Inhalation studies and expertise across 15+ therapeutic areas, Veeda brings both breadth and depth to respiratory drug development.
Global Infrastructure and Technological Innovation
Veeda’s infrastructure reflects the scale necessary for multinational asthma trials.
The company operates 588-bed capacity for healthy volunteer studies and maintains a 30-bed Phase I clinic specifically designed for first-in-human studies.
This infrastructure is complemented by sites across 26 geographies, providing the global reach essential for diverse patient populations in asthma research.
The organization’s technological capabilities align with modern regulatory expectations.
Leveraging AI and advanced real-world data capabilities, Veeda transforms clinical trial management through sophisticated Clinical Trial Management Systems (CTMS) and digital platforms that ensure real-time visibility and regulatory compliance.
Addressing US and European Regulatory Expectations
Regulatory Compliance in the Era of Enhanced Oversight
The FDA’s recent emphasis on electronic systems and data integrity requires CROs to demonstrate advanced technological capabilities and robust quality management systems.
Similarly, the EMA’s focus on Good Clinical Practice (GCP) compliance demands comprehensive documentation and systematic quality assurance.
Veeda addresses these expectations through ICH-compliant operations and adherence to international standards.
The company’s quality management system encompasses comprehensive documentation, electronic data capture systems, and rigorous validation protocols that meet FDA 21 CFR Part 11 compliance requirements and EMA guidelines.
Veeda demonstrates robust capabilities in conducting inhalation studies involving both healthy volunteers and patient populations.
The inhalation studies include bioequivalence and pharmacokinetic assessments of inhalation powders and devices, with a proven track record in two-way crossover PK studies and collaborations with global sponsors for regulatory submissions.
Veeda’s expertise extends to designing and managing studies in patients with respiratory diseases, leveraging a strong network of respiratory physicians and key opinion leaders (KOLs) in pulmonology.
This KOL network enables Veeda to recruit and engage patient cohorts efficiently, facilitate protocol development, and ensure clinical relevance and scientific rigor in respiratory disease research, including asthma, COPD, and other pulmonary conditions.
Overcoming Historical Challenges in Indian Clinical Research
The Indian clinical research industry has faced regulatory challenges, with some CROs experiencing data integrity issues that led to suspension of marketing authorizations.
However, this scrutiny has catalyzed industry-wide improvements. The New Drugs and Clinical Trials (Amendment) Rules, 2024 mandate CRO registration and enhanced oversight, creating a more transparent and accountable research environment.
Veeda’s proactive approach to regulatory compliance positions it favorably in this evolving landscape.
The company’s working relationships global pharmaceutical companies demonstrate its ability to meet international standards.
This track record is particularly significant given that US and European sponsors increasingly seek partners with proven regulatory competency.
Strategic Advantages for Asthma Drug Development
Cost-Effectiveness Without Compromising Quality
Clinical trials in India offer 40-70% cost savings compared to US or EU studies, making it an attractive destination for asthma drug development where extended patient monitoring and specialized equipment requirements can be substantial.
However, these savings extend beyond simple cost arbitrage. Faster patient recruitment due to India’s large and diverse patient population accelerates trial timelines, reducing overall development costs.
For asthma studies specifically, India’s disease burden mirrors that of developed nations, providing access to relevant patient populations with varying severity levels and comorbidity profiles.
The country’s genetic diversity enhances the external validity of clinical trial results, making them more applicable to global populations.
Specialized Capabilities in Complex Respiratory Studies
Asthma drug development presents unique challenges including device-dependent delivery systems, pharmacokinetic complexities, and patient compliance issues. Veeda’s experience with inhalation studies encompasses these critical areas:
Device Compatibility and Validation: Understanding the interaction between drug formulation and delivery devices is crucial for asthma medications.
Veeda’s expertise in bioavailability and bioequivalence studies includes sophisticated testing of inhaler devices and formulation optimization.
Given the complexity of inhalation studies, Veeda ensures that healthy subjects receive guidance on the correct use of inhalation devices.
This approach enhances participant awareness and helps guarantee accurate and reliable study results.
Case Studies and Success Stories in Respiratory Medicine
Advancing COPD and Asthma Treatment Development
Veeda’s contribution to COPD and asthma treatment advancements demonstrates its capability in respiratory drug development.
The company’s role in biomarker-driven research has been particularly significant, as biomarkers are essential for patient stratification and therapy response assessment in asthma trials.
The organization’s expertise in biologics development is especially relevant given the growing importance of monoclonal antibodies in asthma treatment.
The USD 6.21 billion mAbs market in 2024 represents one of the fastest-growing segments in asthma therapeutics, requiring sophisticated analytical capabilities that Veeda provides through its Biopharma division.
Supporting Global Pharmaceutical Innovation
Veeda’s biosimilar development capabilities exemplify its technical sophistication.
Given that several key asthma biologics are approaching patent expiration, biosimilar development represents a significant opportunity requiring precise analytical characterization and clinical validation – areas where Veeda has demonstrated expertise.
The company’s technological platforms including LC-MS, Flow Cytometry, and ELISA provide the analytical foundation necessary for complex asthma drug development, particularly for novel biological entities and combination therapies.
Meeting Future Challenges in Asthma Drug Development
Adapting to Evolving Regulatory Landscapes
The mandatory CRO registration requirements effective April 2025 represent India’s commitment to elevating clinical research standards.
Veeda’s proactive registration and compliance efforts position it advantageously as regulatory authorities implement enhanced oversight mechanisms.
The US BioSecure Act potentially restricting partnerships with certain Chinese entities creates opportunities for Indian CROs like Veeda to expand their relationships with US pharmaceutical companies.
This geopolitical shift, combined with India’s proven regulatory compliance capabilities, enhances Veeda’s strategic position.
Technological Innovation and Digital Transformation
Modern asthma drug development increasingly relies on digital health technologies, remote monitoring, and AI-driven analytics.
Veeda’s investment in digital platforms and AI capabilities aligns with these trends, enabling more efficient trial conduct and enhanced data quality.
The company’s real-time tracking and insights capabilities are particularly valuable for asthma studies where patient adherence and device usage monitoring are critical for successful outcomes.
Strategic Recommendations for Pharmaceutical Partners
Leveraging India’s Clinical Research Advantages
Pharmaceutical companies developing asthma therapeutics should consider India not merely as a cost-reduction strategy but as a strategic research hub offering unique capabilities.
The combination of cost-effectiveness, diverse patient populations, and advanced technical capabilities creates compelling value propositions for asthma drug development.
Regulatory Pathway Optimization: Veeda’s experience with global regulatory submissions can help sponsors navigate complex approval pathways across multiple jurisdictions, reducing development timelines and regulatory risks.
Risk Mitigation Through Strategic Partnership
A successful partnership with Veeda ensures the implementation of robust quality management systems, fosters strong relationships with international clients, and leverages an impressive regulatory track record.
These strengths work together to minimize risks while maximizing development efficiency through diligent oversight and continuous due diligence.
The company’s presence across multiple geographies enables seamless coordination of multinational trials, particularly important for asthma studies requiring diverse environmental and genetic backgrounds.
Conclusion: Positioning for Success in Asthma Drug Development
The rising asthma burden, expanding treatment options, and evolving regulations present both challenges and opportunities for pharmaceutical innovation.
Veeda Lifesciences, with its comprehensive clinical research capabilities, regulatory expertise, and specialized inhalation experience, is an ideal partner for next-generation asthma drug development.
As the asthma therapeutics market grows toward USD 36.49 billion by 2030, partnering with proven CROs like Veeda enables pharmaceutical companies to navigate regulatory complexities, accelerate development, and deliver impactful therapies.
Veeda’s strong track record and India’s clinical research advantages provide a solid foundation for advancing asthma treatments that meet global regulatory standards.
Respiratory drugs play a crucial role in enhancing patients’ quality of life.
Leveraging its expertise in inhalation studies and asthma drug development, Veeda expands access to effective treatments, helping more patients breathe easier and enjoy improved well-being.
References:
- https://projects.gbreports.com/united-states-life-sciences-2024/veeda-clinical-research-company-profile
- https://veedalifesciences.com/inhalation-clinical-trials-challenges-and-ways-to-overcome/
- https://youtu.be/-1tm75J6y0U?si=iotZev0npYquq-pq
- https://www.pwc.in/assets/pdfs/consulting/management-consulting/clinical-trial-opportunities-in-india.pdf
- https://clinicaltrials.gov/study/NCT00980200
- https://www.linkedin.com/pulse/understanding-new-drugs-clinical-trials-amendment-rules-nakrani-ddeaf/
- https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application
- https://www.mordorintelligence.com/industry-reports/asthma-drugs-market
- https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(24)00630-8/fulltext
- https://www.marketresearchfuture.com/reports/asthma-drugs-market-43168
- https://media.market.us/asthma-statistics/
Figure 1: