Introduction: Addressing Critical Healthcare Challenges
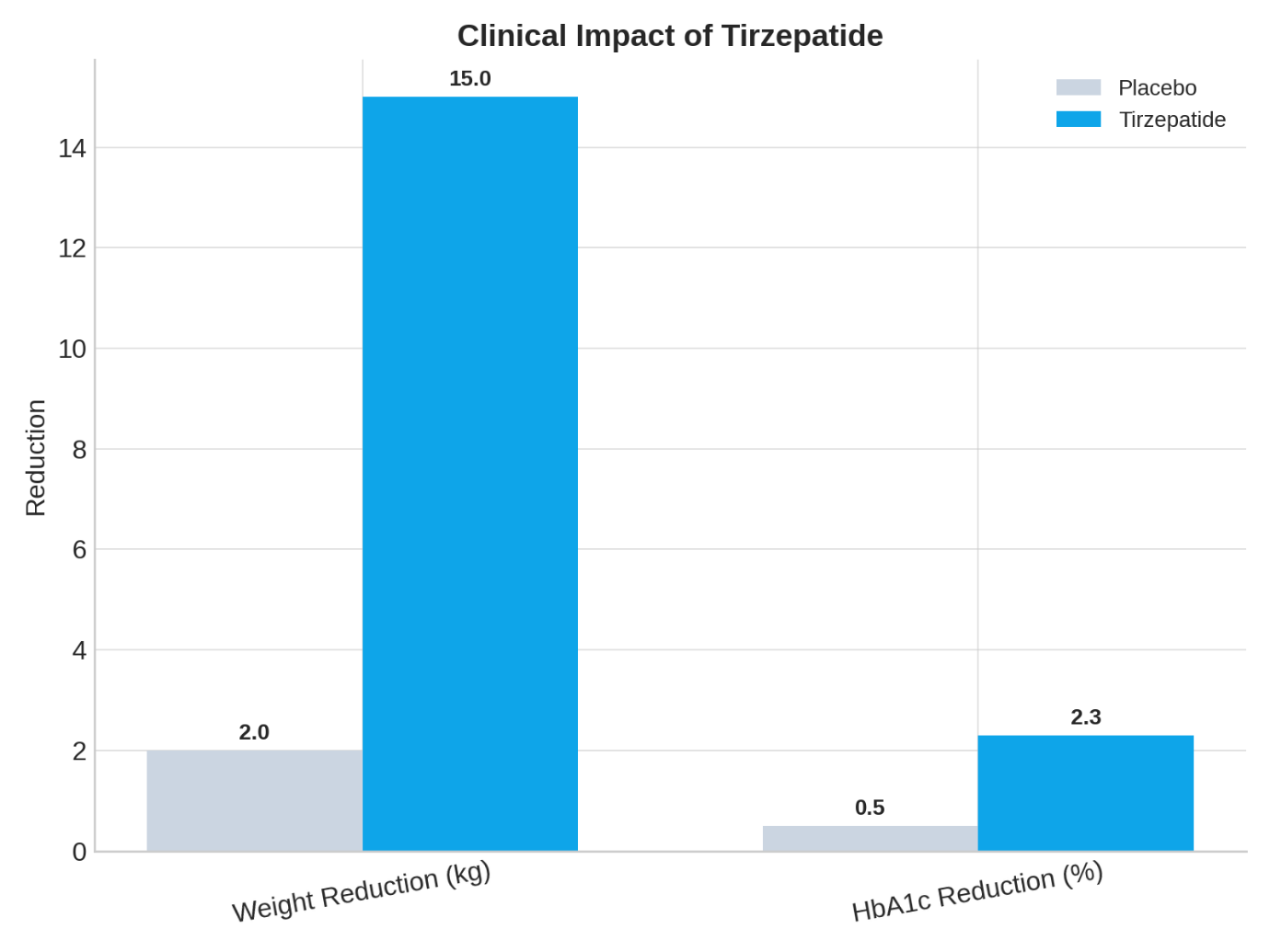
Tirzepatide represents a revolutionary breakthrough in addressing two of the most pressing global health challenges of our time: type 2 diabetes and obesity. As a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, this innovative biotherapeutic has demonstrated unprecedented efficacy in clinical trials, achieving weight reductions of 16.5% to 22.4% over 72 weeks and superior glycemic control compared to existing treatments. The drug’s unique mechanism of action targeting both incretin pathways has positioned it as a game-changing therapy for patients who have not achieved optimal outcomes with conventional treatments.
However, the clinical success of such complex protein therapeutics hinges critically on rigorous analytical characterization during development and manufacturing. This is where Veeda Lifesciences’ Biopharma Division has established itself as a leader, employing cutting-edge imaged Capillary Isoelectric Focusing (icIEF) technology to ensure the highest standards of quality and consistency in biotherapeutic development.
The Critical Importance of Charge Variant Analysis in Biotherapeutics
Charge heterogeneity represents one of the most fundamental Critical Quality Attributes (CQAs) for protein therapeutics like Tirzepatide. Post-translational modifications including deamidation, oxidation, glycosylation, and other chemical changes can significantly alter a protein’s charge profile, directly impacting its safety, efficacy, pharmacokinetics, and immunogenicity.
For Tirzepatide specifically, maintaining consistent charge profiles is essential because:
- Therapeutic efficacy: Charge variants can affect receptor binding affinity and biological activitym
- Safety profile: Altered charge states may increase immunogenic potential
- Regulatory compliance: Regulatory agencies require comprehensive charge variant profiling for approval
- Manufacturing consistency: Batch-to-batch reproducibility depends on controlling charge heterogeneity
The isoelectric point (pI) determination becomes particularly crucial as it influences protein solubility, stability, and aggregation behavior. Understanding and controlling these parameters is essential for developing robust formulations that maintain drug product integrity throughout storage and administration.
Veeda’s Advanced icIEF Technology: Setting New Standards
Custom Method Development for Tirzepatide
Veeda’s Analytical and Characterization team has developed a tailored icIEF methodology specifically optimized for Tirzepatide’s unique physicochemical properties. This custom approach demonstrates the company’s deep understanding that one-size-fits-all analytical methods are inadequate for complex biotherapeutics. The method development process involved systematic optimization of:
- Sample preparation protocols to maintain protein integrity
- Voltage and temperature parameters for optimal separation
- Capillary conditioning procedures to ensure reproducible results
- Detection wavelengths for maximum sensitivity
Dual-Marker Strategy for Enhanced Accuracy
A key differentiator in Veeda’s approach is the implementation of a dual-marker strategy using pI markers at 3.38 and 5.85. This sophisticated calibration system ensures:
- Accurate pI determination across the relevant pH range
- Robust method validation with traceable reference standards
- Enhanced precision in charge variant quantification
- Regulatory compliance with industry best practices
This approach surpasses conventional single-marker methods by providing multiple reference points that bracket the target protein’s expected pI range, significantly improving analytical accuracy and reliability.
Fluorescence-Based Detection: Superior Resolution
Veeda’s choice of fluorescence-based detection over traditional absorbance methods represents a significant technological advancement. This approach delivers:
- Enhanced resolution for closely spaced charge variants
- Improved signal-to-noise ratios enabling detection of minor variants
- Greater sensitivity for low-concentration samples
- Reduced interference from sample matrix effects
The superior performance of fluorescence detection is particularly critical for charge variant profiling, where the ability to resolve and quantify minor species can be the difference between a successful and failed analytical method.
Technical Excellence: Demonstrating Analytical Rigour
High Reproducibility Across Multiple Injections
The analytical robustness of Veeda’s icIEF method is exemplified by the high reproducibility achieved across triplicate injections for each sample. This level of precision is essential for:
- Regulatory submissions requiring demonstrated method reliability
- Quality control applications in manufacturing environments
- Stability studies tracking charge variant changes over time
- Comparability assessments between different batches or formulations
Software-Driven Analysis with Compass Integration
The utilization of Compass software for data acquisition and integration demonstrates Veeda’s commitment to state-of-the-art analytical infrastructure. This sophisticated data management system provides:
- Automated peak integration reducing human error
- Standardized reporting formats ensuring consistency
- Comprehensive data archiving for regulatory compliance
- Advanced statistical analysis capabilities for method validation
System Suitability Validation
The implementation of five-marker system suitability checks showcases Veeda’s dedication to analytical excellence. While these markers were not used in final pI calculations, their inclusion demonstrates:
- Comprehensive method validation beyond minimum requirements
- Proactive quality assurance measures
- Analytical method robustness under varying conditions
- Professional analytical practices aligned with industry standards
Addressing Unmet Medical Needs: The Tirzepatide Impact
Revolutionary Dual-Pathway Mechanism
Tirzepatide’s unprecedented dual agonism of both GIP and GLP-1 receptors represents a paradigm shift in metabolic disease treatment. This innovative mechanism delivers:
- Superior glycemic control with HbA1c reductions of up to 2.34%
- Remarkable weight loss exceeding traditional GLP-1 agonists
- Cardiovascular benefits including reduced stroke and mortality risk
- Potential neuroprotective effects against dementia and neurodegeneration
Global Health Impact
The development of Tirzepatide addresses critical unmet medical needs affecting hundreds of millions of patients worldwide:
- Type 2 diabetes: Over 400 million adults globally require more effective treatments
- Obesity epidemic: Approximately 70% of American adults are overweight or obese
- Cardiovascular disease: Leading cause of mortality linked to metabolic disorders
- Healthcare economics: Reducing long-term complications and healthcare costs
Clinical trials have demonstrated that Tirzepatide enables many patients to achieve ≥20% weight loss, a threshold previously attainable primarily through bariatric surgery. This breakthrough offers a non-invasive therapeutic option for patients with severe obesity who may not be surgical candidates.
icIEF Technology: The Gold Standard for Charge Analysis
Technological Advantages Over Traditional Methods
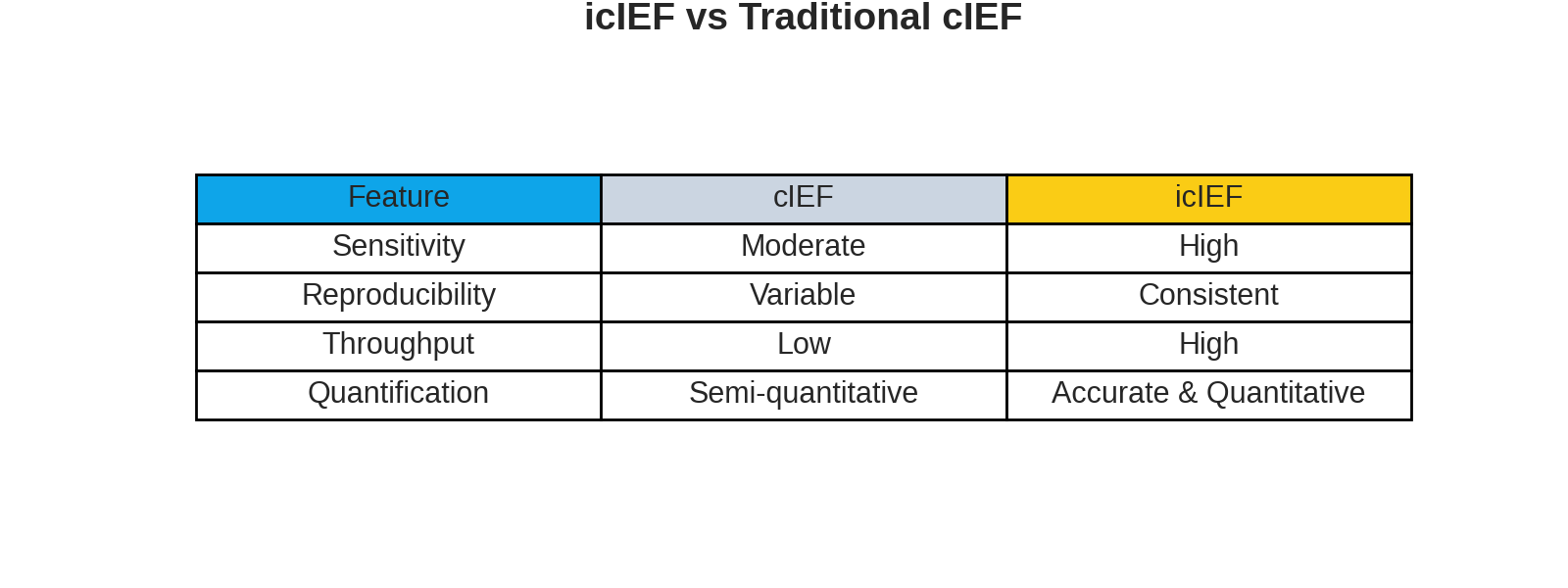
Imaged Capillary Isoelectric Focusing has emerged as the gold standard for biopharmaceutical charge variant analysis. Compared to conventional capillary isoelectric focusing (cIEF), icIEF offers:
- Faster separation times eliminating the mobilization step
- Higher resolution enabling detection of subtle charge differences
- Improved reproducibility through real-time imaging
- Simplified method development reducing time-to-results
- Enhanced throughput for high-volume applications
The technology utilizes a CCD camera to image the entire capillary length simultaneously, providing instantaneous detection without the complications associated with traditional mobilization procedures.
Regulatory Acceptance and Compliance
The widespread adoption of icIEF across the pharmaceutical industry reflects its regulatory acceptance and validation capabilities. Key advantages include:
- ICH guideline compliance for method validation
- Proven track record in regulatory submissions
- Standardized protocols across multiple organizations
- Quality control applications in GMP environments
Advanced Coupling with Mass Spectrometry
Modern icIEF systems can be directly coupled to high-resolution mass spectrometry, enabling:
- Simultaneous separation and identification of charge variants
- Molecular weight determination for variant characterization
- Post-translational modification mapping
- Real-time structural analysis during separation
This icIEF-MS hyphenation represents the cutting edge of analytical biotechnology, providing unprecedented insight into protein heterogeneity.
Veeda’s Competitive Differentiators
Integrated Biopharma Platform
Veeda Lifesciences offers a comprehensive integrated platform spanning the entire drug development lifecycle:
- Discovery biology and target identification
- Bioprocess development for protein production
- Analytical characterization using advanced techniques
- Clinical bioanalysis supporting clinical trials
- Regulatory submissions with global expertise
This integrated approach eliminates traditional development bottlenecks and ensures seamless project progression from research through commercialization.
Global Regulatory Experience
With over 80 successful regulatory audits and submissions to agencies worldwide, Veeda brings invaluable regulatory expertise to biotherapeutic development. This experience is particularly crucial for complex products like Tirzepatide, where regulatory pathways require comprehensive analytical data packages.
State-of-the-Art Infrastructure
Veeda’s investment in cutting-edge analytical instrumentation and GMP-compliant facilities provides clients with:
- High-resolution mass spectrometry capabilities
- Advanced biophysical characterization techniques
- Automated analytical workflows for increased throughput
- Data integrity systems ensuring regulatory compliance
Scientific Excellence and Innovation
The company’s commitment to scientific innovation is demonstrated through:
- Method development expertise for novel biotherapeutics
- Advanced analytical techniques beyond standard methodologies
- Publication record in peer-reviewed journals
- Industry collaboration with leading pharmaceutical companies
Future Perspectives: Advancing Biotherapeutic Development
Emerging Analytical Technologies
Veeda continues to invest in next-generation analytical platforms including:
- Native mass spectrometry for structural characterization
- Multi-dimensional separations for complex heterogeneity profiling
- Automated high-throughput systems for increased capacity
- AI-driven data analysis for enhanced interpretation
Expanding Therapeutic Applications
The success with Tirzepatide positions Veeda to support development of other complex biotherapeutics including:
- Antibody-drug conjugates requiring specialized analytical approaches
- Bi-specific antibodies with unique characterization challenges
- Gene therapies demanding novel analytical strategies
- Cell therapies requiring advanced potency assessments
Global Market Leadership
Veeda’s proven expertise in advanced analytical characterization positions the company for continued growth in the expanding global biopharmaceutical market. The increasing complexity of new therapeutic modalities will drive demand for sophisticated analytical capabilities like those demonstrated in the Tirzepatide project.
Conclusion: Excellence in Analytical Sciences
Veeda Lifesciences’ successful implementation of advanced icIEF technology for Tirzepatide characterization exemplifies the company’s position as a leader in biopharmaceutical analytical sciences. The project’s success—characterized by optimized methodology, robust reproducibility, and comprehensive charge variant profiling—demonstrates how sophisticated analytical approaches are essential for developing life-changing therapies.
The differentiators achieved by Veeda’s Analytical and Characterization team, including custom method development, dual-marker calibration strategies, fluorescence-based detection, and comprehensive system suitability testing, represent the gold standard for analytical excellence in biotherapeutic development.
As Tirzepatide continues to transform the treatment landscape for diabetes and obesity, addressing critical unmet medical needs for millions of patients worldwide, the analytical foundation provided by technologies like icIEF ensures that this breakthrough therapy meets the highest standards of quality, safety, and efficacy. Veeda’s proven capabilities in this challenging analytical domain position the company to continue supporting the development of tomorrow’s life-saving biotherapeutics.
The integration of cutting-edge analytical technologies with deep scientific expertise and regulatory experience makes Veeda Lifesciences an ideal partner for pharmaceutical companies developing complex biotherapeutics. As the industry continues to push the boundaries of therapeutic innovation, the analytical characterization capabilities demonstrated in projects like Tirzepatide will remain fundamental to bringing safe and effective treatments to patients in need.
References:
- https://www.nature.com/articles/s41366-024-01488-5
- https://www.cureus.com/articles/172909-safety-and-effectiveness-of-tirzepatide-use-in-obesity-without-type-2-diabetes-mellitus
- https://www.mdpi.com/2227-9059/11/6/1667
- https://diabetesjournals.org/diabetes/article/73/Supplement_1/297-OR/155363/297-OR-A-Novel-GLP-1-FGF21-Dual-Agonist-ZT003-Has
- https://www.mdpi.com/2227-9059/12/1/159
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9438179/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9747662/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9268041/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11445313/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10088547/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11704219/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10923294/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11044191/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11211290/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10213833/
- https://www.jomes.org/journal/view.html?doi=10.7570%2Fjomes22067
- https://en.wikipedia.org/wiki/Tirzepatide
- https://pubmed.ncbi.nlm.nih.gov/33325008/
- https://www.creative-proteomics.com/pronalyse/pi-determination-with-ief.html
- https://www.britannica.com/science/tirzepatide
- https://go.drugbank.com/drugs/DB15171
- https://linkinghub.elsevier.com/retrieve/pii/S0731708522005994
- https://www.semanticscholar.org/paper/af006a5bebb0ed9520d4591499ce591eb1333d5b
- https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/elps.201100611
- https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jssc.201200633
- https://xlink.rsc.org/?DOI=D4AY00836G
- https://www.ssrn.com/abstract=4213342
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11564561/
- https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/elps.201900325
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10092839/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10070887/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6235248/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10256444/
- https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/elps.202300170
- https://www.hindawi.com/journals/jamc/2023/8150143/
- https://www.frontiersin.org/articles/10.3389/fchem.2025.1536222/full
- https://www.agilent.com/cs/library/primers/public/5991-1660EN.pdf
- https://pubmed.ncbi.nlm.nih.gov/22736354/
- https://samsungbiologics.com/media/science-technology/improving-therapeutic-protein-efficacy-through-charge-profile-adjustment
- https://www.thermofisher.com/in/en/home/industrial/pharma-biopharma/biopharmaceutical-analytical-testing/intact-protein-analysis-workflows/charge-variant-analysis.html
- https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/elps.201100611
- https://isogen-lifescience.com/_file/2280/iCIEF_employing_FC_and_MC_coated_fused_silica_capillary.pdf
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10860345/
- https://pubmed.ncbi.nlm.nih.gov/36435084/
- https://www.chromatographyonline.com/view/charge-variant-profiling-biopharmaceuticals
- https://jrtdd.com/index.php/journal/article/view/3536
- https://www.jsad.com/doi/10.15288/jsad.23-00343
- https://ijocp.com/index.php/IJOCP/article/view/124
- http://www.ectrx.org/detail/archive/2021/19/7/0/651/0
- https://www.tandfonline.com/doi/full/10.1080/15504263.2016.1254309
- https://ascopubs.org/doi/10.1200/JCO.2022.40.28_suppl.102
- https://www.semanticscholar.org/paper/475f223e4301c919f46d7353eba9f6254bfddd05
- https://dx.plos.org/10.1371/journal.pmen.0000142
- https://www.journalslibrary.nihr.ac.uk/ghr/published-articles/JVNW9009
- https://journaljammr.com/index.php/JAMMR/article/view/5404
- https://assets.cureus.com/uploads/original_article/pdf/242476/20240410-32508-1dqqmja.pdf
- https://assets.cureus.com/uploads/editorial/pdf/234318/20240323-5554-lq9785.pdf
- https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2024.1424570/pdf
- https://www.frontiersin.org/articles/10.3389/fmed.2024.1346208/pdf?isPublishedV2=False
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11544576/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC5488177/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9865573/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11457899/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10990713/
- http://www.researchprotocols.org/2020/1/e14744/
- https://projects.gbreports.com/india-life-sciences-2023/veeda-clinical-research-interview
- https://www.piramalpharmasolutions.com/resources/blogs/analytical-development-in-pharma-essential-for-safe-effective-drug-manufacturing
- https://aktinos.in/analytical-development-services/
- https://www.nice.org.uk/guidance/gid-ta10835/documents/674
- https://zenvisionpharma.com/service/analytical-development/
- http://progenericspharma.com/services/analytical-development/index.html